The Connected in Motion (CIM) team was fortunate enough to participate in the 83rd Scientific Sessions of the American Diabetes Association, one of the world’s premier platforms dedicated to advancing diabetes research and care. We’re excited to share with you the insights and knowledge gained from these thought-provoking sessions. Our aim is to ensure that the diabetes community in Canada benefits from these global discussions, helping all of us better understand the disease, manage it more effectively, and enhance our overall health and quality of life.
There’s exciting news on the horizon! Vertex Pharmaceuticals Incorporated has recently shared positive findings from an ongoing clinical trial of VX-880, a groundbreaking treatment that utilizes stem cell-derived islet cells. This innovative therapy aims to restore insulin production and improve blood sugar control in individuals with Type 1 diabetes. Let’s delve into the details presented during the Vertex session, and explore how this development could potentially transform the lives of people with T1D!
Understanding Stem Cell-Derived Islet Cells
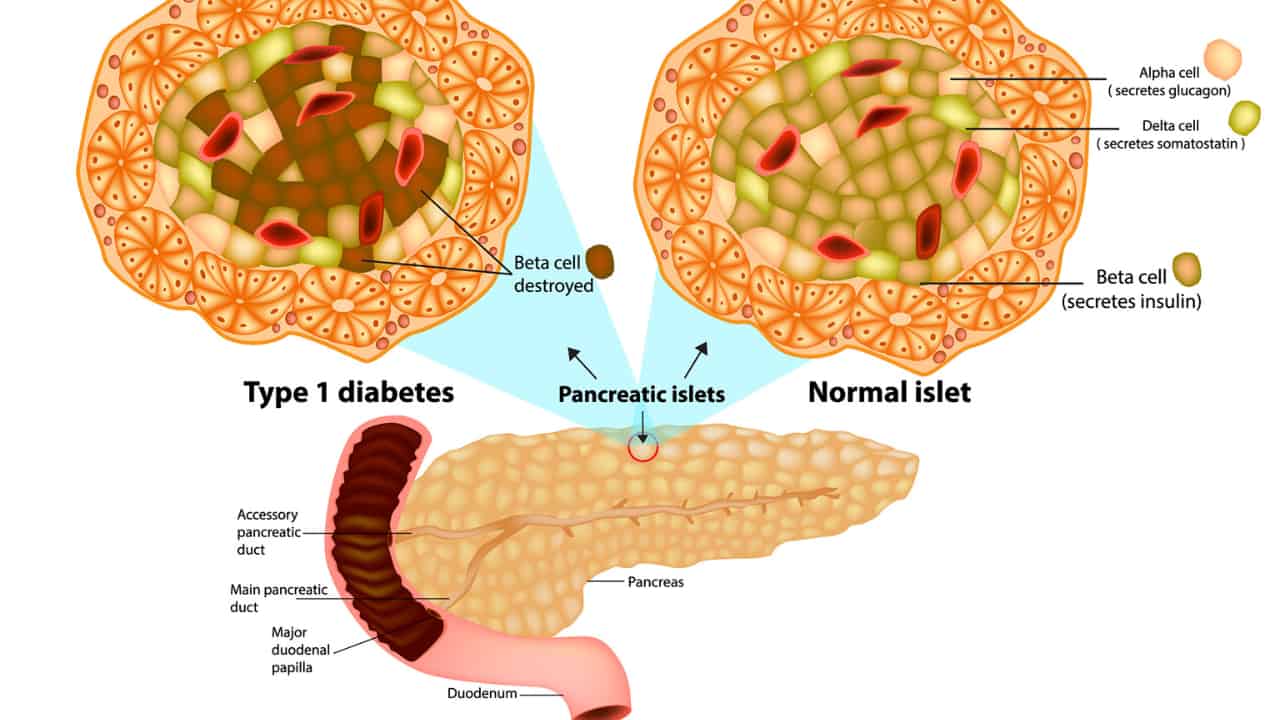
To grasp the significance of VX-880, it’s essential to understand stem cell-derived islet cells. These specialized cells are generated from stem cells, which possess the remarkable ability to transform into various cell types in our bodies. In the case of stem cell-derived islet cells, scientists manipulate stem cells in the laboratory to create cells resembling the islet cells found in the pancreas. Islet cells are responsible for producing insulin, the hormone that regulates blood sugar levels. By leveraging these stem cell-derived islet cells, researchers aim to restore the natural ability of the body to produce insulin in people with Type 1 diabetes.
Positive Results from the Clinical Trial
The recent clinical trial of VX-880, presented at the American Diabetes Association’s 83rd Scientific Sessions, focused on individuals with Type 1 diabetes experiencing impaired hypoglycemia awareness and severe hypoglycemic events. Unlike current treatment options like exogenous insulin, which do not address the underlying causes of the disease, this study explored the potential of stem cell-derived islet cell therapy as a future treatment avenue.
The trial included six participants with undetectable insulin secretion and a history of recurrent severe hypoglycemic events. After receiving VX-880 treatment, all six patients exhibited restored insulin secretion, improved glycemic control, reduced or eliminated exogenous insulin usage, and experienced no severe hypoglycemic events during the 90-day evaluation period. These encouraging outcomes suggest that stem cell-derived islet cell therapy holds significant promise as an effective treatment for Type 1 diabetes.
Two patients who received VX-880 for over 12 months achieved insulin independence and demonstrated remarkable improvements in glycemic control. Their HbA1c levels were below the diagnostic threshold for diabetes, and more than 95% of their blood glucose readings fell within the desired range, surpassing the recommended target set by the American Diabetes Association. These results are truly remarkable considering the daily challenges faced by individuals living with Type 1 diabetes.
Additional patients treated with the full target dose of VX-880 for up to 90 days also showed positive outcomes, including insulin production, reduced HbA1c levels, improved time-in-range, and decreased daily insulin usage. These findings align with the positive results observed in patients treated for over one year.
Safety and Next Steps
The safety profile of VX-880 has been generally favorable, with only mild or moderate adverse events reported. Importantly, there were no serious adverse events related to VX-880 treatment.
Based on the positive safety and efficacy data, an independent data review committee has recommended advancing to Part C of the trial. This next phase will allow concurrent dosing of patients at the full target dose of VX-880. Excitingly, the trial has expanded to include additional sites in Norway, Switzerland, and the Netherlands, providing more opportunities for individuals living with Type 1 diabetes to participate and potentially benefit from this groundbreaking therapy.
The Promise of Stem Cell-Derived Islet Cells
These remarkable findings offer great hope to the Type 1 diabetes community. Stem cell-derived islet cells have the potential to become a future treatment option, revolutionizing the management of Type 1 diabetes. Imagine a world where exogenous insulin administration is no longer necessary to achieve optimal blood sugar control. Although further research and development are needed, this breakthrough development could significantly impact the lives of those living with Type 1 diabetes, offering newfound freedom and improved quality of life.
In conclusion, the ongoing clinical trial of VX-880 utilizing stem cell-derived islet cells brings us closer to a potential breakthrough in Type 1 diabetes treatment. This innovative therapy has shown promising results in restoring insulin production and enhancing blood sugar control. As the research progresses, we remain hopeful for a future where individuals with Type 1 diabetes can experience improved health and well-being, free from the constraints of exogenous insulin dependence.





