In February 2020, Connected in Motion got a sneak peek at Abbott’s FreeStyle Libre 2 at the Advanced Technologies & Treatments for Diabetes Conference. The FreeStyle Libre 2 CGM system was approved by the US FDA in June 2020 and by Health Canada in December 2020. The Libre 2 is similar to the original FreeStyle Libre system in many ways but has added a few neat features. Read on to learn what’s new about the Libre 2!

New Features
Bluetooth Sensor and Optional Alerts
The Libre 2 sensor has Bluetooth connectivity that allows it to alert users with a sound or vibration if their BG is high or low in real-time without scanning. The alert triggers users to scan their sensor with a reader to see their actual BG number and make any necessary adjustments. These alerts are customizable and optional.

Approved for Children
Another exciting piece of news is that the FreeStyle Libre 2 CGM system was approved for children ages 4 and up by the FDA and Health Canada. With the addition of alerts, the Libre 2 can help kids with diabetes and their parents and caregivers feel more safe and secure.
Integration Potential
While the FreeStyle Libre and Libre 2 are considered flash glucose monitoring in Canada, the Libre 2 received Integrated Continuous Glucose Monitoring (iCGM) designation from the US FDA, allowing it to be used with other devices like insulin pumps and glucose meters. The Libre 2 cannot currently be used with any automated insulin delivery systems. Promisingly, at the Advanced Technologies & Treatments for Diabetes Conference, Abbott announced they were partnering with Insulet to work on the Omnipod Horizon insulin pump that will be compatible with the Libre 2.
Maintained Features
Receiver
Both the original FreeStyle Libre and Libre 2 are designed to be scanned with a compatible reader and/or an app. The original FreeStyle Libre has the “FreeStyle LibreLink” app and the Libre 2 has the “FreeStyle Libre 2” app to scan the CGM sensor and obtain BG information.
NOTE: In the US, the reader is currently required for use to scan the Libre 2 sensor and view glucose readings. The FreeStyle Libre 2 app is available in Canada and under review by the US FDA.
Price
The Libre 2 is available at the same price as the original FreeStyle Libre system. Abbott advertises the FreeStyle Libre 2 system as one-third of the price of other CGM systems, depending on insurance coverage.
Sensor Design
Both the original FreeStyle Libre and Libre 2 have easy-to-apply sensors that are the size of two stacked quarters and can be worn on the back of the upper arm for 14 days.

Frequency of BG Reading
Both the original FreeStyle Libre and Libre 2 measure BG every 1 min.
Sensor Memory
After scanning both the original FreeStyle Libre and Libre 2, the reader provides glucose readings from the last 8 hours. With both systems, the sensor has to be scanned in order to obtain BG information, making them considered flash technology.
Warm-up Time and Calibration
Both the original FreeStyle Libre and Libre 2 have 1 hour warm-up times and neither require calibrations.
Below you will find a handy chart summarizing these differences and similarities between the FreeStyle Libre and FreeStyle Libre 2:
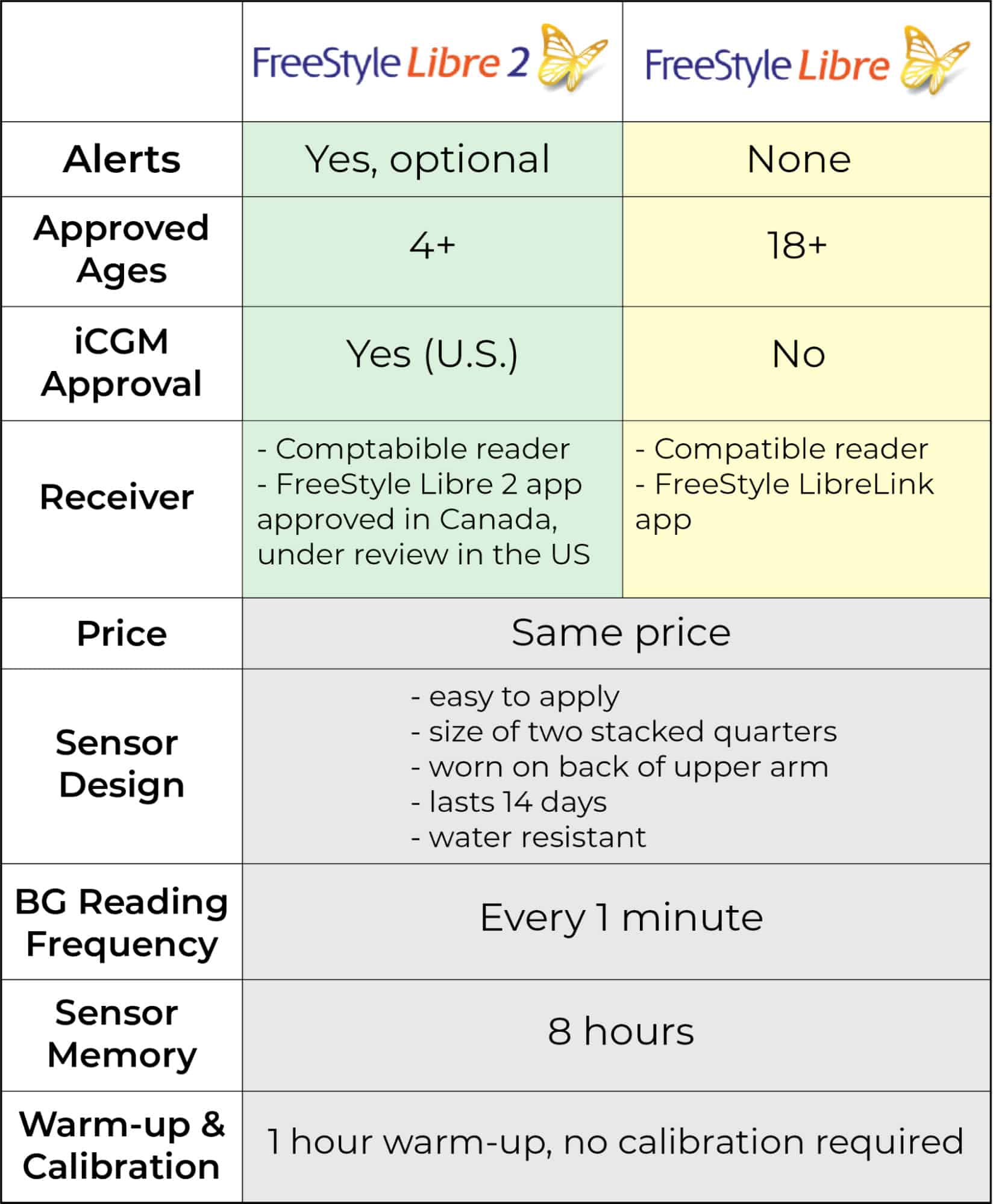
Adapted from: https://www.freestyle.abbott/us-en/compare-cgms.html FreeStyle Libre CGM Systems vs. Dexcom Table
Diabetes tech updates are always exciting, but sometimes it can be hard to compare and contrast new technology with existing devices. We hope this blog helped clarify the new Libre 2 features for you!





